Capturing Dragons - A fiery photographic experiment.
An experiment in photographing flame at extreme shutter speeds. Propane torch tests at 1/8000s, these frames freeze fire into sharp, sculpted motion.

An experiment in photographing flame at extreme shutter speeds. Propane torch tests at 1/8000s, these frames freeze fire into sharp, sculpted motion.

During my years working in the sign industry, I spent a lot of time around fire.
At first, I did a bit of everything. But as the business grew and more staff were employed, I moved into the specialist role of mould maker. I’d mark everything out by hand using 1:1 scale plots, then build the moulds and any parts that needed curves, like the letter S, D, and anything with serifs.
Traditionally, you’d use a purpose-built oven to heat 100mm-wide, 3mm acrylic strips, however here, we used propane torches instead. Much faster but far more temperamental. Heat it unevenly and it warps. Move too slowly and it blisters or catches fire. Once it blisters, it’s basically a write off: it releases nasty fumes, crackles and pops as it burns and the piece is ruined. Doing it well takes years of practice.

During those years, I became comfortable around fire — around flammable gas, and even the occasional gas leak. Compared to the YouTube fail-compilation panic of someone screaming at a small kitchen flame, I felt at home standing there with twin propane torches blasting intense heat like miniature jet engines, right up near my face.

A propane torch is simple, mechanically speaking: a regulator sets pressure from the bottle, gas runs through the line to the handle, and a valve controls flow. When it’s lit, the nozzle draws in air through vents and mixes it with the gas to create a tight, hot blue flame.
In simpler terms: valve on, spark = jet engine in my hands.
That blue flame — brutally hot, roughly 20 - 40cm long — had to be moved quickly and evenly over the acrylic to avoid burning it.
Every now and then, purely for the drama of it (and no, don’t do this), I’d partially block the air intake. I wore heat-resistant gloves in that workshop anyway, because we handled hot acrylic all day. But the effect was instant: the clean blue “rocket” became a much larger orange flame — the kind you’d expect to see at the top of an oil refinery flare stack.

Giant orange flames upwards of 1 meter high dancing around and the heat against your face. It was mesmerising.
One day after work — with the boss’s permission — I decided to try capturing that dance at the highest shutter speed my camera could manage: 1/8000th of a second. Tripod, 10-second timer, blocked off the air vents and shot away. Back then I shot in black and white. The images were RAW (so the colour data was still there), but I liked judging tone and composition without colour getting in the way. Honestly, I still edit that way: contrast and exposure first, then bring the colour back in later.

What happens if i introduce more flammable fluids like Methylated Spirits into the mix. Rather than do this in the air and risk burning down the work shed. I cleared a large area of concrete floor, with a bucket of water and a fire extinguisher just in case it got out of hand. I poured water all over the floor, mainly to reduce heating of the concrete or potential cracking, and the propane torch laying on the floor with some weights to hold it in place. I then used some thin makeshift tin to cover the air vents, now with giant orange flame flowing, I proceed to pour methylated spirits into the already roaring flame.
The experiment didn't last long, just 5 mins or so.
But the results are spectacular.
In one shot it appears as if a hooded wraith rides atop an ethereal dragon. In others, you can see the individual droplets of methylated spirits before before it ignites, and the reflections rippling across the wet concrete. Even in daylight, the extreme shutter speed reduced the background to near-black and let the flame expose correctly — bright and sharp.

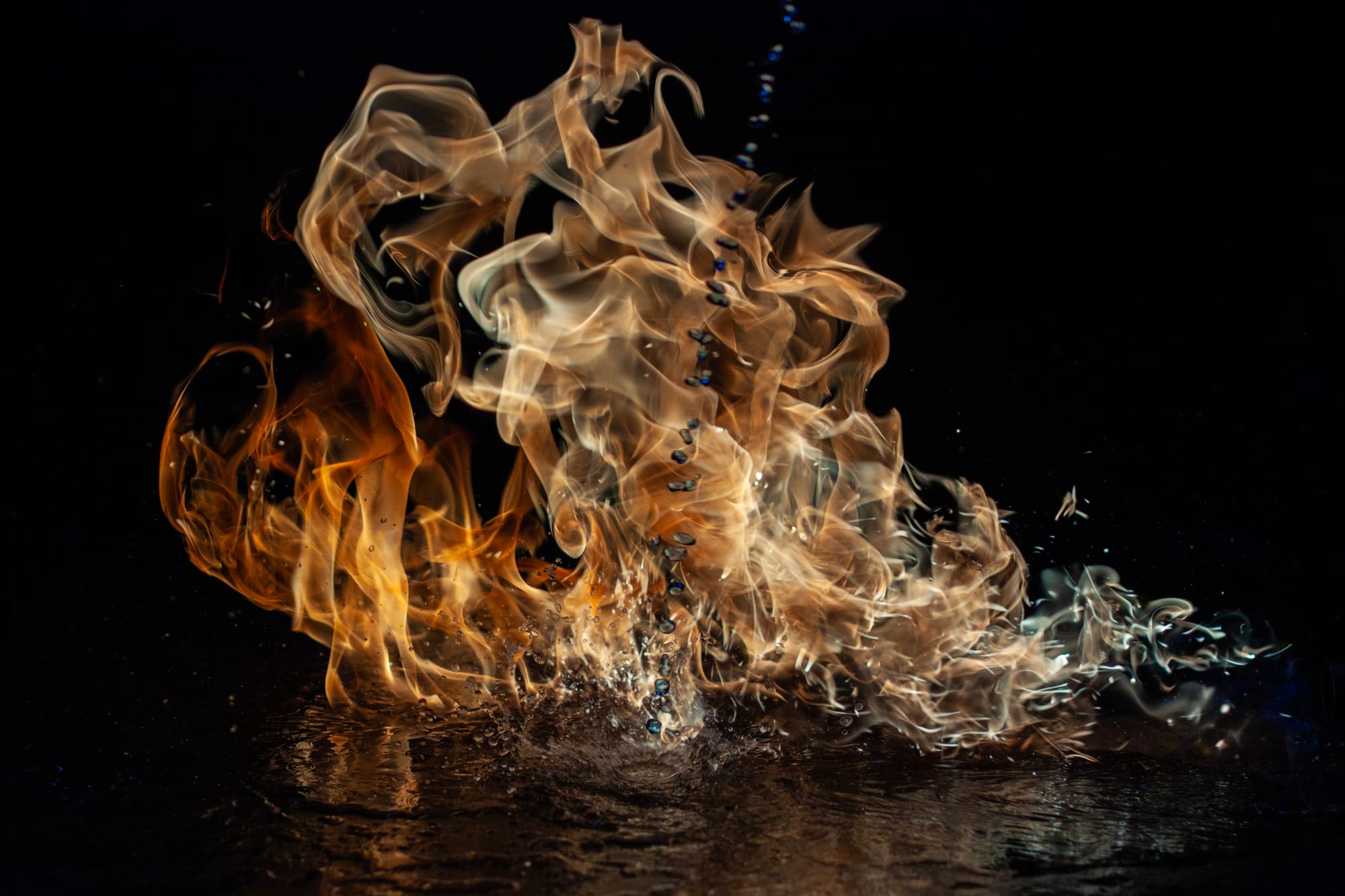
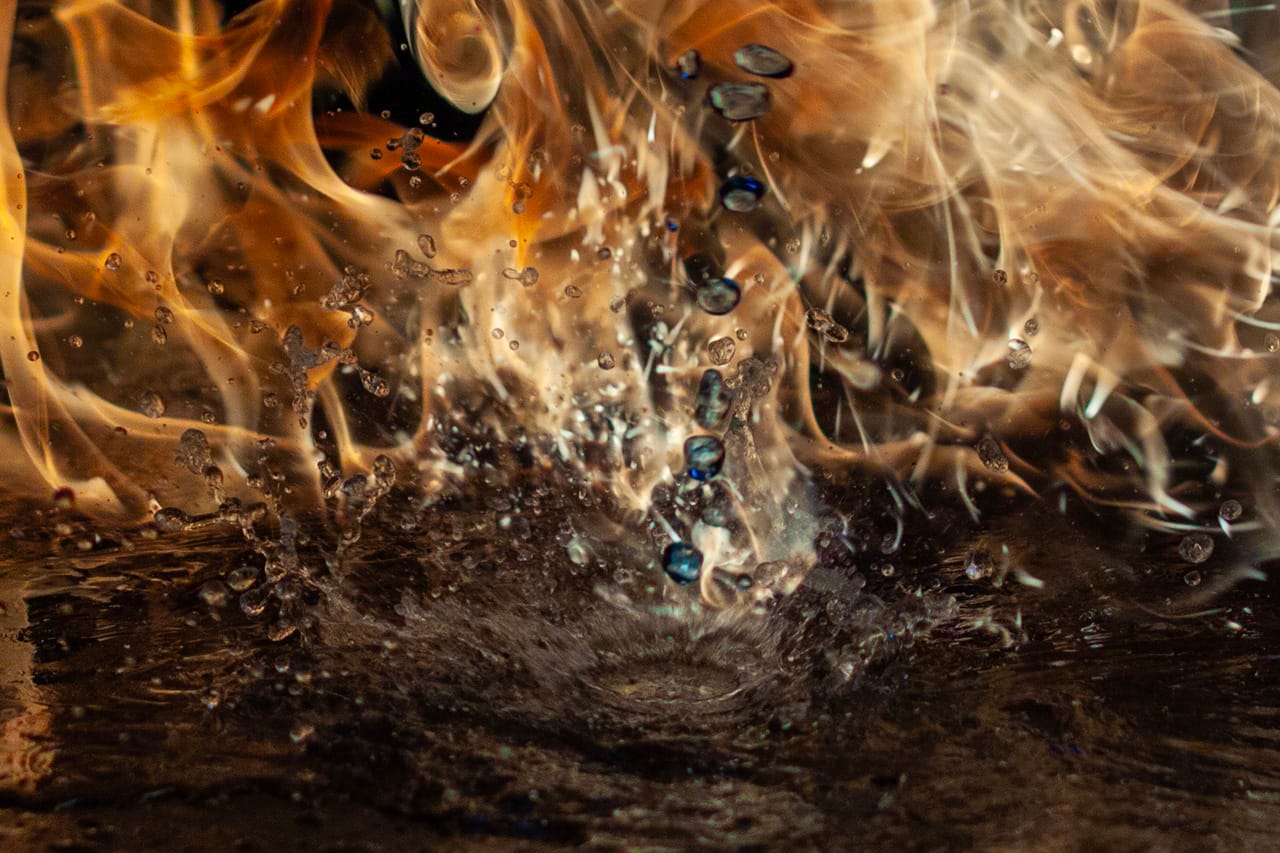

I ended up with more than 350 images. A lot of the early “torch-in-hand” shots later became layer elements in photo-composite work — little pieces I could use as texture or effects.
TECH NOTES: Some images have been manipulated to show more depth. The overwhelming red is reduced by dragging the raw image colour temperature deep into the blue (around -95 or raw 2400k ), which removes most of the colour information. Then a vibrance and saturation increase is required to bring back the colour. The image at the top of this article is straight off camera with standard RAW conversion, colour temperature and saturation at zero.
The camera used for this experiment was a Canon 50D and a Canon 50mm f1.8 (nifty fifty) all of which was quite cheap at the time. (November 2009)
DISCLAIMER: Please do not play with flammable gas in this way. I have extensive experience working with flammable gas and have undergone fire safety training. I am aware the risks and had many safety precautions in place, more than have been explained in this article. Flammable gas is capable of exploding and causing serious injury and or death.







The Fire Experiment Gallery
Everything on this site is free for everyone.
No ads. No popups. No paywalls.
Subscribe to receive the free monthly newsletter, and you’ll be able to comment on posts.
If you’d like to help keep this project sustainable, there are Supporter and Sponsor tiers available - Totally optional.